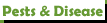I found an article on hydrobuilder.com (
https://hydrobuilder.com/learn/npk-rati ... t-balance/) which explains this rather well. There's only one limitation -- the article applies to hydroponic crop production, not growing ornamental plants like cacti and succulents. As we go through the following quotes, I'll comment on each one, pointing out things that are specific to cacti and succulents:
- "Nitrogen is responsible for producing robust, leafy growth. This is why you’ll see it in higher ratios during veg, and it will fall off during flower."
Not true for ornamentals -- N is used with the same ratio all the time. Also, N promotes stem growth in cacti and leaf growth in succulents.
- "Phosphorus does the opposite, helping your plants develop flowering sites and produce large buds. Traditionally, growers would ramp up the phosphorus during flower because of this."
Actually, not the best explanation because it applies only to crops, so here's a better one from The Spruce (
https://www.thespruce.com/):
- "Phosphorus plays a key role in the growth of roots, blooming, and fruiting, which is why it is an essential nutrient for your plants in spring. Phosphorus contributes to many fundamental plant processes, such as rooting and seed formation."
Not just in spring -- P is essential for cacti and succulents throughout the entire growing season. Now back to Hydrobuilder:
- "Potassium, on the other hand, isn’t necessarily veg or bloom specific. Instead, it helps regulate a variety of plant functions, including strong root growth, increased resistance to disease and increased water intake, along with thick, sturdy growth."
Definitely true for
all plants.
- "Magnesium and calcium go hand in hand, and are often an area where plants become deficient. Magnesium helps aid in the uptake and utilization of other nutrients, along with producing carbs and sugars to help during flower."
Once again, definitely true for
all plants.
- "Calcium has a similar role in plants as in humans, helping produce strong cells and root walls. This leads to stronger plants. We've recently come to understand that calcium is actually the dominant nutrient in most plants." [My emphasis]
Quite a revelation, and it backs up a couple of things MikeInOz has said in previous posts -- "there's no such thing as too much Ca" and "there's no such thing as Ca toxicity". In fact, I offer the possibility that Ca higher than N could be a
very good thing at least for cacti and succulents.
- "Sulfur helps produce chlorophyll, and plays an essential role in foliage and root development."
Applies to cacti and succulents too. Sulfur deficiency = chlorosis, and yellowing of the skin indicates a serious S deficiency. If you have a good amount of sulfate in your watering solution, chlorosis won't be a problem.
This brings us to...

- Mulders_chart.jpg (87.83 KiB) Viewed 4064 times
- "This shows how nutrients interact with one another. Nutrients interact either antagonistically or synergistically.
They can increase the uptake of one another. Or, they will fight among each other and can lock each other out if the balance/ratio is off."
Precisely why NPK ratios are key to knowing if your fertilizer is right or wrong for cacti and succulents. The ideal NPK ratio = 1:0.25-0.35:1.1-1.7. Simply put -- of the 3 primary major nutrients, ornamentals need the least amount of P. If your feeding regimen has the ideal ratio, all other nutrients are there in sufficient amounts, and you don't overdo it on the fertilizer (plus any supplements you may need), your plants will be happy, healthy and growing well over the years. (Many thanks to SDK1 for posting Mulder's Chart on 2/1/2023.)