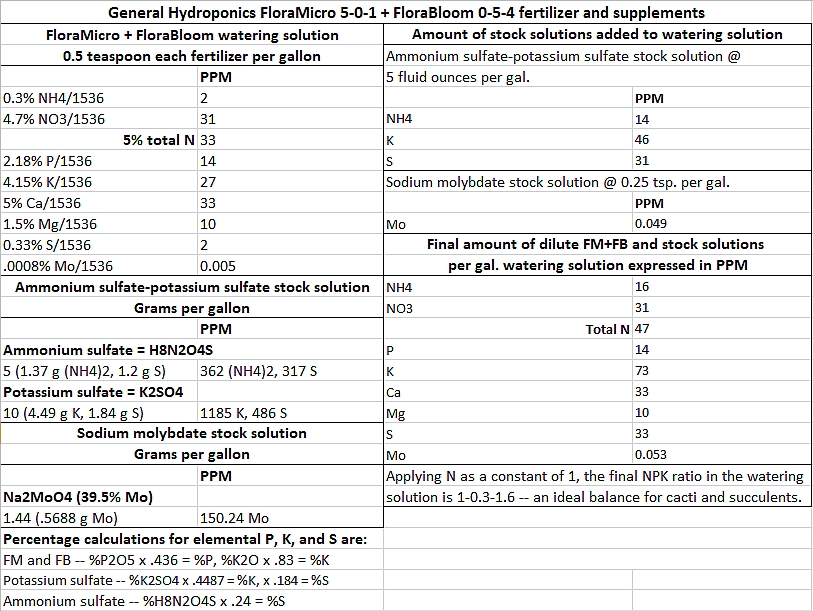
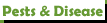jerrytheplater wrote: ↑Fri Mar 10, 2023 3:54 amSteve, I hope I can explain this without confusing you or anyone else. The term "Nitrate-Nitrogen" is a term used when looking at lab reports or fertilizer bags. No chemical exists that is called Potassium Nitrate-Nitrogen. It would be Potassium Nitrate KNO3. As it dissolves it splits into two ions: Potassium K+ and Nitrate NO3-. Not Nitrate-Nitrogen ion NO3-N-. That last ion does not exist in reality. Nitrate-Nitrogen just tells you that the Nitrogen being talked about is derived from Nitrate ions only.
The nitrogen in Potassium Nitrate can be analyzed and reported as % Nitrate or % Nitrate-Nitrogen. Both numbers are describing the same compound. Both numbers can be used to calculate how much is in the bag of fertilizer. You just have to make sure you know what the manufacturer is saying.
What you said is quite understandable, so all I needed was an explanation -- which you just did. Thanks.
With that in mind, Davide PM'd me about a fertilizer he's considering in place of Ferty 3:
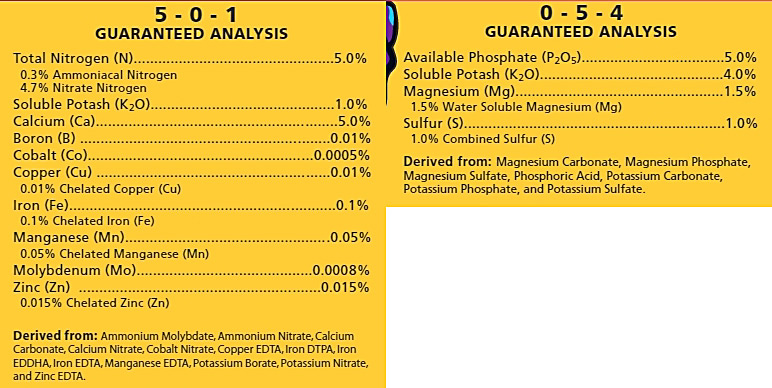
Cifo Idrofloral Tech 15-10-30 (1-0.29-1.66)
Total nitrogen (N) 15%,
Nitric nitrogen (N) 8.5%,
Nitrogen (N) ammoniacal 4%,
Nitrogen (N) urea 2.5%,
Phosphorus pentoxide (P2O5) soluble in neutral ammonium citrate and in water 10%,
Phosphorus pentoxide (P2O5) soluble in water 10%,
[Not sure why it's listed twice.]
Potassium oxide (K2O) soluble in water 30%,
Sulfur trioxide (SO3) soluble in water 5%,
Boron (B) soluble in water 0.02%,
Copper (Cu) soluble in water 0.02%,
Copper (Cu) chelated with EDTA 0.02%,
Iron (Fe) soluble in water 0.05%,
Iron ( Fe) chelated with EDTA 0.05%,
Manganese (Mn) soluble in water 0.02%,
Manganese (Mn) chelated with EDTA 0.02%,
Molybdenum (Mo) soluble in water 0.01%,
Zinc (Zn) water soluble 0.02%,
Zinc (Zn) chelated with EDTA 0.02%
This is a dry fert, and I'm recommending 0.4 grams per liter for the watering solution. If you and Mike could do an evaluation including the ppm numbers, it'll tell us whether or not Davide will be getting what I think he's getting -- a fert with everything all in one bag, no stock solution required. All that's missing is Ca, but he can add limestone or gypsum to his mix depending on species.